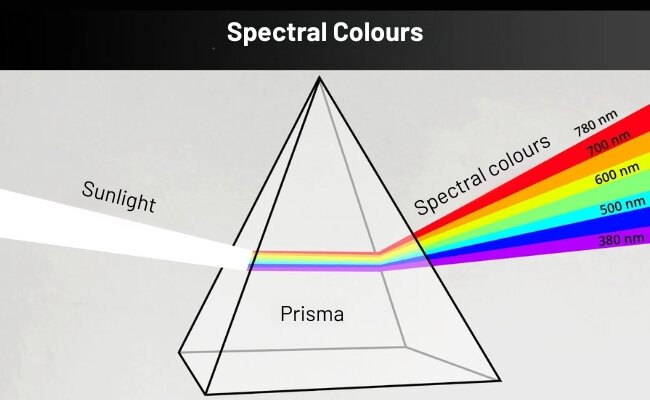
Spectral colours are the colour impression of a hue with a wavelength between 380 and 780 nanometres that is visible to the human eye - they are known as rainbow colours and are created when white light is refracted through a prism.

Checkout using your account
Checkout as a new customer
Creating an account has many benefits:

Spectral colours are the colour impression of a hue with a wavelength between 380 and 780 nanometres that is visible to the human eye - they are known as rainbow colours and are created when white light is refracted through a prism.
| Colour tone | wavelength in nm |
| Violet | 380 to 430 nanometre |
| Blue | 430 to 490 nanometre |
| Green | 490 to 570 nanometre |
| Yellow | 570 to 600 nanometre |
| Orange | 600 to 640 nanometre |
| Red | 640 to 780 nanometre |
The first person to scientifically document the breaking up of light into spectral colours in 1676 was Isaac Newton. He used a prism and allowed sunlight to fall on it, whereupon the light was broken down into different colours. There are actually around 300 spectral colours that can be distinguished by humans; however, the term is often only associated with the rainbow colours red, orange, yellow, green, blue, indigo and violet. Incidentally, the term "rainbow colours" was not chosen by chance. It is closely related to the phenomenon of light refraction through a prism: The sun provides white light; the raindrops act as a prism and thus ensure that the light is broken down into its spectral colours.
When white light hits a prism, the spectral colours are created, but what happens when the spectral colour hits a prism again? Does the human eye recognise new colour changes? Spectral colours are pure colours, i.e. they cannot be broken down into other colours. If the experiment with the prism were repeated with spectral colours instead of white light, no new colours would be created.